GMP Peptide Synthesis
- The peptide can be used for biological function analysis, but also has a high biological activity, low toxicity, high specificity and other inherent characteristics. The uses of peptides in clinical applications are increasing every year. Many of peptides are already marketed as drugs, and some are in different stages of clinical development. When implemented in the early stages of peptide drug development, custom cGMP peptide synthesis can help expedite the maturation of your peptide drug.
Our GMP Service Package Includes
- Process development
- Impurity study
- Isolation and identification of known and unknown impurities
- Analytical development and characterization
- Analytical validation
- Full stability studies
- Process validation
- Drug Master Files (DMFs)
- Regulatory support
- Preparation and characterization of an in-house reference standard
Our Standard cGMP Specifications Include
- Appearance
- Solubility
- Identity
- Peptide purity
- Related substances
- Assay
- Bacterial endotoxins
- Counterion content
- Water
- Mass balance
- Specific rotation
- Residual organic solvents
Highlight
Technology Platform
- Raw material qualification
- Synthetic method development, validation and scale up
- Product purification
- GMP/GLP analytic method development and validation
- Release specification design and testing
- GMP packaging
GMP Compliance
- On-going training
- In-house audit
- Raw material control
- Analytical method validation and documentation
- Standard operating procedures (SOPs)
- In process control and batch record
Certificate of Analysis for GMP Peptides
- Appearance and solubility
- Purity by HPLC
- Heavy metal analysis by ICP-MS
- Bioburden or microbial tests
- Residual organic solvent by GC
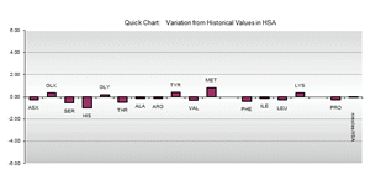
- Amino acid analysis
- Molecular Weight by MS
- Water content
- Endotoxin
- Peptide content (nitrogen estimation)
- Counter ion content (% TFA) by IC
-
After-sales Service
ABclonal provides a free-issue retest or refunds for the unqualified products within 2 weeks. Provide 1 to 3 month free retesting for ineligible items and 1 to 2 weeks free store for samples.
-
Technical Support
ABclonal provides one-to-one technical support service for each customer.
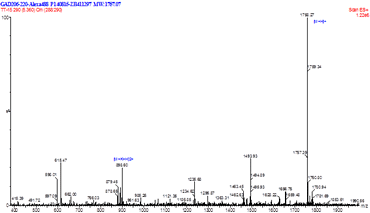
Certificate of analysis includes MS and HPLC analyses. These analyses are followed by independent QA (quality assurance) procedures, guaranteeing the highest quality of peptides.
Analysis List
- Chromatography (HPLC, TLC, GC, IC)
- Chromatography coupled to MS (LC-MS/ESI)
- Spectroscopy (UV-Vis, IR, NMR)
- Refractometry
- Polarimetry
- Metallic Analysis (AAS, ICP, ICP-MS)
- Elemental Analysis
- Titration (Acid-Base Titration, Karl Fischer Titration)
- Amino acid analysis