Basic Information
| Clone | Evolocumab Biosimilar |
|---|
| Molecular Weight | 150 kDa |
|---|
| Endotoxin | <1EU/mg (<0.001EU/μg)Determined by LAL gel clotting assay |
|---|
| Sterility | 0.2 μm filtration |
|---|
| Aggregation | <5% Determined by SECP |
|---|
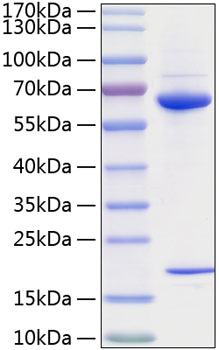
| Purity | >95% Determined by SDS-PAGE |
|---|
Product Information
| Production | Purified from cell culture supernatant in an animal-free facility |
|---|
| Purification | Protein A or G purification |
|---|
| Storage | 2 - 8°C for up to 4 weeks and -80°C for long term storage (Avoid repeated freezing and thawing) |
|---|
Target Background
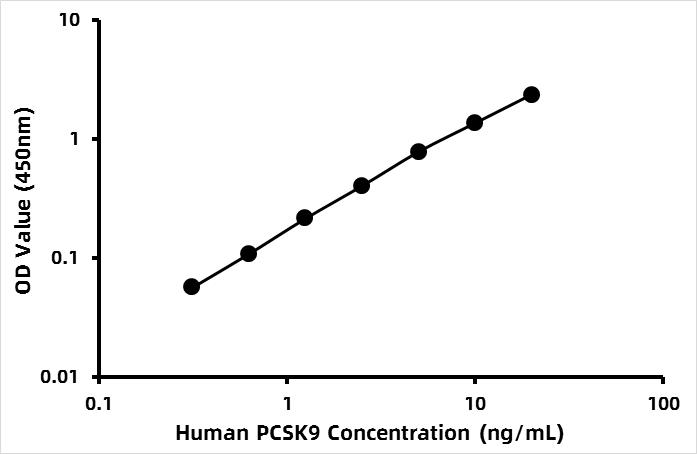
What is evolocumab biosimilar research grade? Evolocumab (trade name Repatha) is a fully human monoclonal antibody that inhibits proprotein convertase subtilisin–kexin type 9 (PCSK9) and lowers low-density lipoprotein (LDL) cholesterol levels by approximately 60%. The PCSK9 protein targets LDL receptors for degradation. Evolocumab binds to the circulating PCSK9 protein, inhibiting it from binding to the LDLR, in turn preventing PCSK9-mediated LDLR degradation and permitting the LDLR to recycle back to the liver cell surface. By inhibiting the binding of PCSK9 to LDLR, evolocumab increases the number of LDLRs available to clear LDL from the blood, thereby lowering LDL-C levels. Evolocumab biosimilar uses the same protein sequences as the therapeutic antibody evolocumab.
Immunogen Information
| Isotype | Human IgG2 lambda |
|---|
| Immunogen | Human PCSK9 |
|---|
| Recommended Dilution Buffer | 1×PBS pH 7.0 |
|---|
* For research use only. Not for therapeutic or diagnostic purposes.

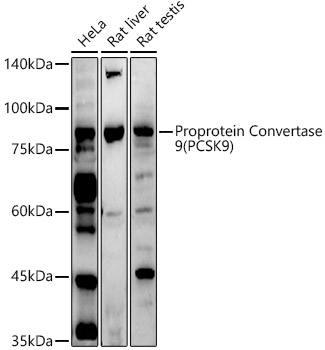
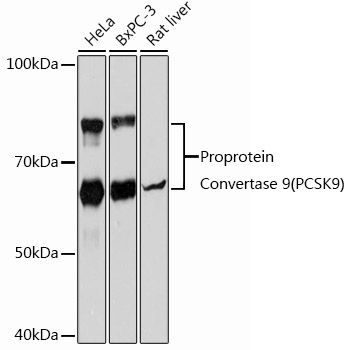
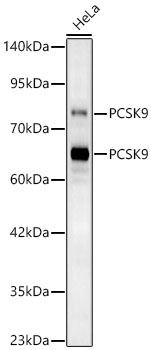
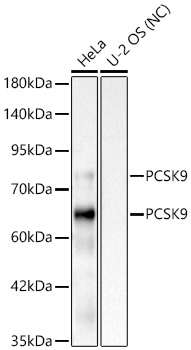
![[KD Validated] Proprotein Convertase 9(PCSK9) Rabbit mAb](https://img.abclonal.com/abclonal-manage/Catalog/A11532/A11532_1.jpg?t=1725348552)