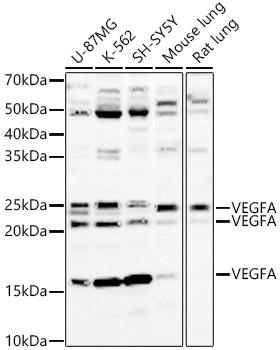
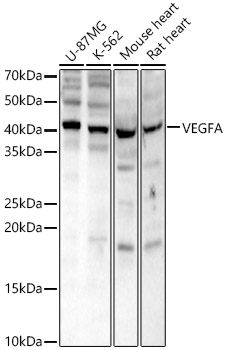
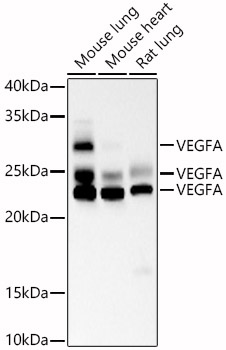
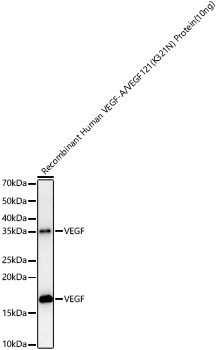
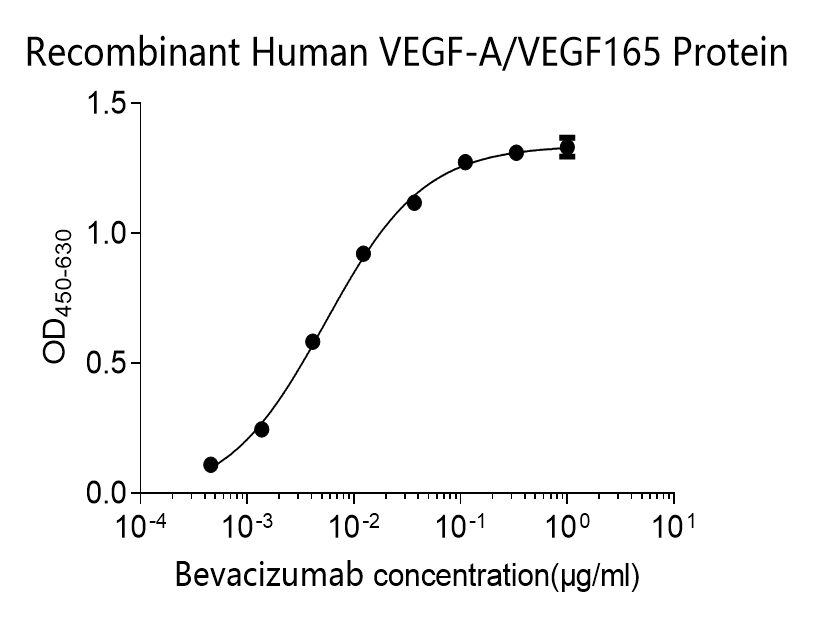
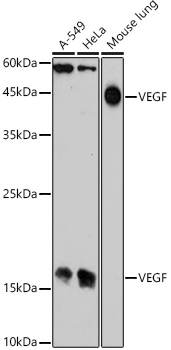
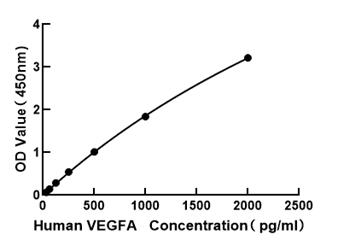
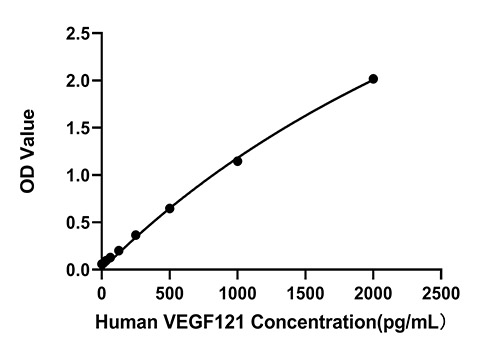
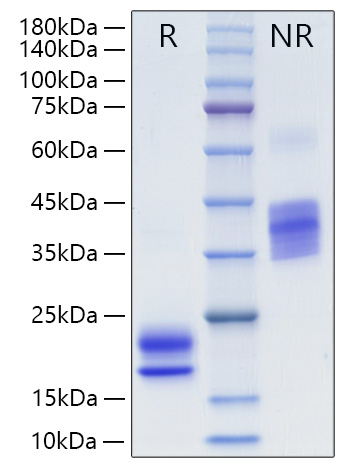
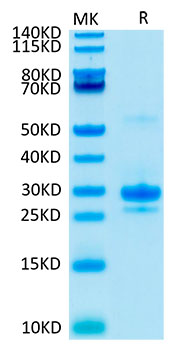
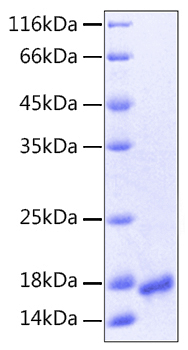
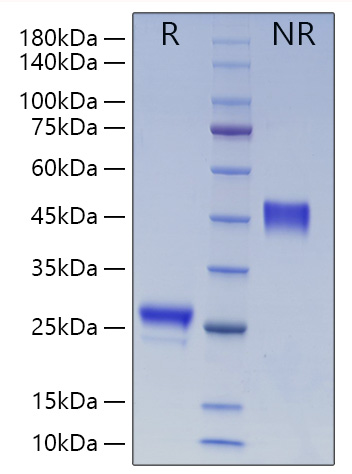
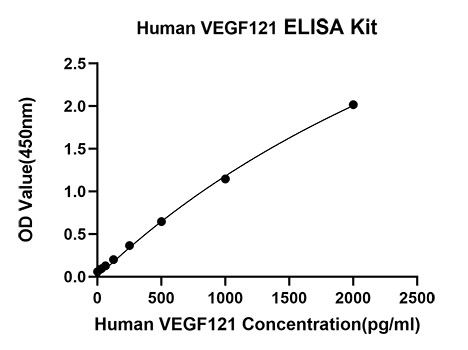
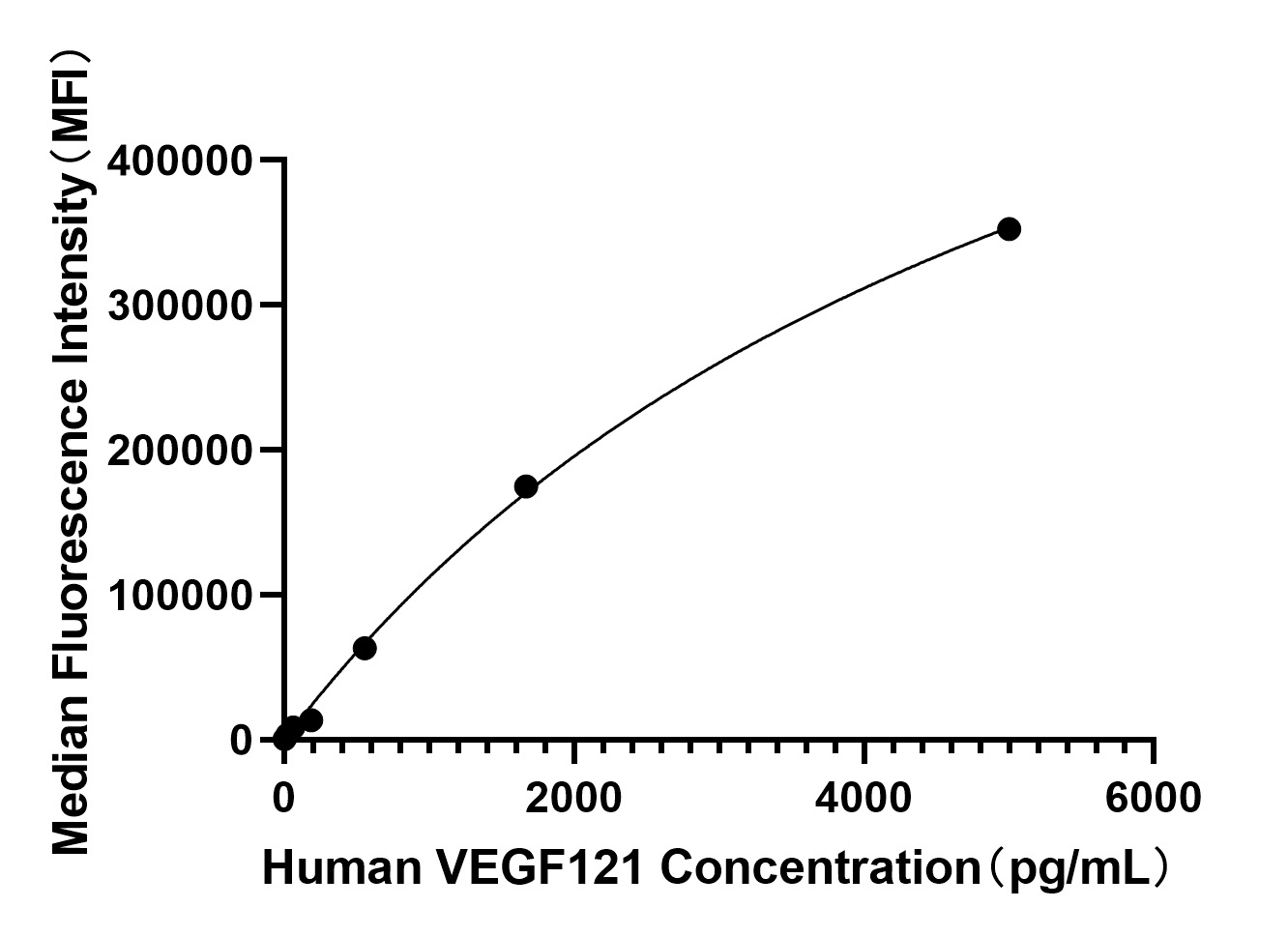
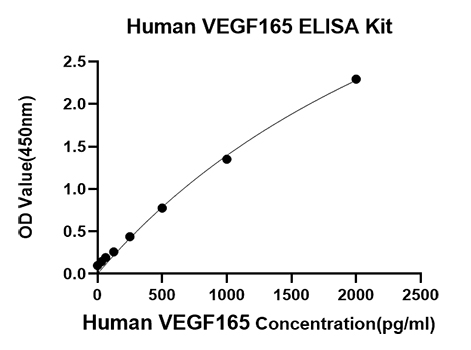
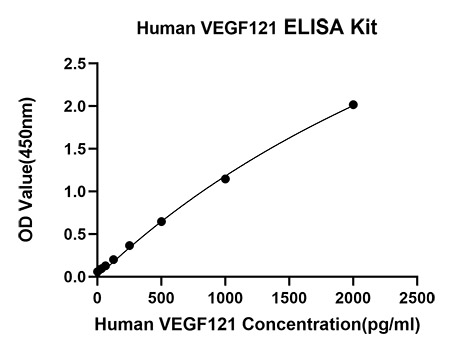
The humanized anti-VEGF-A monoclonal antibody drug Bevacizumab (trade name Avastin, Genentech/Roche) is the first clinically available angiogenesis inhibitor in the United States. The humanized anti-VEGF-A monoclonal antibody fragment (Fab) Ranibizumab (trade name Lucentis, Genentech) is derived from the same parent mouse antibody as bevacizumab. Both antibody drugs produce angiogenesis inhibition and slow the growth of new blood vessels. Ranibizumab is much smaller than the parent complete antibody but shows stronger binding to VEGF-A after affinity maturation. Ranibizumab can also be used to treat the "wet" type of age-related macular degeneration (AMD, also ARMD), a common form of age-related vision loss.Vascular endothelial growth factor A (VEGF-A) stimulates angiogenesis in a variety of cancers, including colorectal, lung, breast, glioblastoma, kidney, and ovarian cancers.